Collaborative project
Transforming SAE reporting in clinical trials with Pharmacovigilance Tracker (PV Tracker)
The Pharmacovigilance Tracker is a web-based software built to streamline the SAE reporting process to improve efficiency without risk to safety

Active
January 2016
Overview
In Clinical Trials of Investigational Medicinal Products (CTIMPs), a Serious Adverse Event (SAE) is any untoward medical occurrence that results in death, life-threatening events, hospitalisation, persistent disability, or congenital anomalies. SAEs must be reported to the sponsor within 24 hours of the study team's awareness, TASC is the Tayside Medical Science Centre at the University of Dundee and is responsible for overseeing adverse event reporting on CTIMPs, including SAEs, within the University of Dundee and/or NHS Tayside.
Challenge
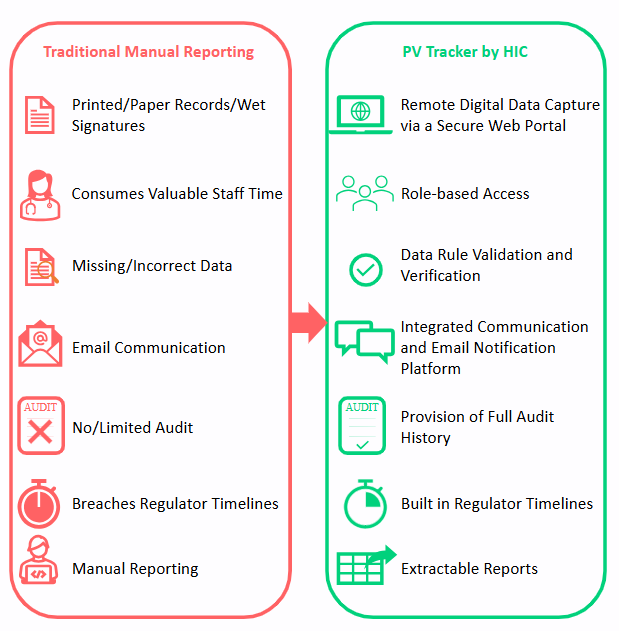
SAE reports were previously completed manually on paper-based forms. These would then be scanned into a computer, e-mailed to the PV monitor that would check for any issues including missing or invalid data. Once necessary changes were complete the report would be scanned again and only at that point, would be ready for medical review.
This cumbersome processes involves various manual tasks and is time consuming. Given the increase in number of CTIMPS being sponsored, TASC felt there was a need for a new purpose-built PV tracking system and partnered with HIC to develop the PV Tracker.
HIC's role
HIC’s software team, in collaboration with TASC, clinical researchers and medical reviewers, developed the PV Tracker. This web-based software streamlined the SAE reporting process to improve efficiency without risk to safety. The features include built-in data validation and regulator timelines, as well as an automated notification framework. Reporting and monitoring can now be done remotely by multiple users by study/site with much greater structure thanks to report grouping and classification. This all helps users keep track of progress, reduces errors and significantly reduces manual efforts. The PV tracker was launched in April 2023 and is currently being used in various clinical trials.
Impact
- Reduced delays in SAE reporting process
- Previous manual tasks now automated
- Multiple users can access reports remotely
- Significant benefits to patient safety
- Streamlined processes, built-in regulator timelines and reduced possibility of human error
- Easier monitoring of reports
- Positive feedback from users
- Used in 6 clinical trials with ~240 users
What's next
The HIC software team are looking to further improve the system by adding the ability for MedDRA (Medical Dictionary for Regulatory Activities) coding to be done and installing an option to export report data in a format that would allow it to be automatically uploaded to the relevant regulatory body for SUSAR (Suspected Unexpected Serious Adverse Reaction) reporting.
HIC has developed a costing model for making the tool a licensed piece of software that can be rolled out to other units, at an affordable cost.
PV Reviewer and Clinical Researcher
Please contact [email protected] to find out more about PV tracker.